One hundred years after the discovery of “cold light,” the presence of
phosphorus in plants and animals was ascertained, and its form was
established as a compound of phosphoric acid. This knowledge had little
practical effect until the “nature” of the acid, in its various forms,
was explained through the work of Thomas Graham. From it, there started
a considerable technical development.
At about that time (1833), the Duke of Richmond proved that the fertilizing value of bones resided not in the gelatin, nor in the calcium, but in the phosphoric acid. Thus, he confirmed what Théodore de Saussure had said in 1804, that “we have no reason to believe” that plants can exist without phosphorus. Unknowingly at first, the farmer had supplied this element by means of the organic fertilizers he used: manure, excrements, bones, and horns. Now, with the value of phosphorus known, a search began for mineral phosphates to be applied as fertilizers. Jean Baptiste Boussingault (1802-1887), an agricultural chemist in Lyons, traveled to Peru to see the guano deposits. Garcilaso de la Vega (ca. 1540 to ca. 1616) noted in his history of Peru (1604) that guano was used by the Incas as a fertilizer. Two hundred years later, Alexander von Humboldt revived this knowledge, and Humphry Davy wrote about the benefits of guano to the soil. Yet, the application of this fertilizer developed only slowly, until Justus Liebig sang its praise. Imports into England rose and far exceeded those into France where, between 1857 and 1867, about 50,000 tons were annually received.
The other great advance in the use of phosphatic plant nutrients started with Liebig’s recommendation (1840) to treat bones with sulfuric acid for solubilization. This idea was not entirely new; since 1832, a production of a “superphosphate” from bones and sulfuric acid had been in progress at Prague. At[Pg 186] Rothamsted in 1842, John Bennet Lawes obtained a patent on the manufacture of superphosphate. Other manufactures in England followed and were successful, although James Muspratt (1793-1886) at Newton lost much time and “some thousands of pounds” on Liebig’s idea of a “mineral manure.”
 It was difficult enough to establish the efficacy of bones and
artificially produced phosphates in promoting the growth of plants under
special conditions of soils and climate; therefore, the question as to
the action of phosphates in the growing plant was not even seriously
formulated at that time. The beneficial effects were obvious enough to
increase the use of phosphates as plant nutrients and to call for new
sources of supply. Active developments of phosphate mining and treating
started in South Carolina in 1867, and in Florida in 1888.[25]
It was difficult enough to establish the efficacy of bones and
artificially produced phosphates in promoting the growth of plants under
special conditions of soils and climate; therefore, the question as to
the action of phosphates in the growing plant was not even seriously
formulated at that time. The beneficial effects were obvious enough to
increase the use of phosphates as plant nutrients and to call for new
sources of supply. Active developments of phosphate mining and treating
started in South Carolina in 1867, and in Florida in 1888.[25]
In a reciprocal action, more phosphate application to soils stimulated increasing research on the conditions and reactions obtaining in the complex and varying compositions called soil. The findings of bacteriologists made it clear that physics and chemistry had to be amplified by biology for a real understanding of fertilizer effects. After 1900, for example, Julius Stoklasa (1857-1936) pointed out that bacterial action in soil solubilizes water-insoluble phosphates and makes them available to the plants.[26]
 The insight into the importance of phosphorus in organisms, especially
since Liebig’s time, is reflected in the work of Friedrich Nietzsche
(1844-1900). This “re-valuator of all values” who modestly said of
himself: “I am dynamite!” once explained the human temperaments as
caused by the inorganic salts they contain: [Pg 187]“The differences in
temperament are perhaps caused more by the different distribution and
quantities of the inorganic salts than by everything else. Bilious
people have too little sodium sulfate, the melancholics are lacking in
potassium sulfate and phosphate; too little calcium phosphate in the
phlegmatics. Courageous natures have an excess of iron phosphate.” (See
volume 12 of Nietzsche’s Works, edit. Naumann-Kröner, Leipzig, 1886.)
In this strange association of inorganic salts with human temperaments,
the role of iron phosphate as a producer of courage is particularly
interesting. What would a modern philosopher conclude if he followed the
development of insight into the composition and function of complex
phosphate compounds in organisms?
The insight into the importance of phosphorus in organisms, especially
since Liebig’s time, is reflected in the work of Friedrich Nietzsche
(1844-1900). This “re-valuator of all values” who modestly said of
himself: “I am dynamite!” once explained the human temperaments as
caused by the inorganic salts they contain: [Pg 187]“The differences in
temperament are perhaps caused more by the different distribution and
quantities of the inorganic salts than by everything else. Bilious
people have too little sodium sulfate, the melancholics are lacking in
potassium sulfate and phosphate; too little calcium phosphate in the
phlegmatics. Courageous natures have an excess of iron phosphate.” (See
volume 12 of Nietzsche’s Works, edit. Naumann-Kröner, Leipzig, 1886.)
In this strange association of inorganic salts with human temperaments,
the role of iron phosphate as a producer of courage is particularly
interesting. What would a modern philosopher conclude if he followed the
development of insight into the composition and function of complex
phosphate compounds in organisms?
The method used in this inquiry was to subject anatomically separated parts of the organisms to chemical separations. The means for such separations had to be more gentle than the strong heat and destructive chemicals that had been considered adequate up to then. The interpretation of the new results naturally relied on the general advance of chemistry, the development of new methods for isolating substances of little stability, of new concepts concerning the arrangements of atoms in the molecules, and of new apparatus to measure their rates of change.[Pg 188]
 [Pg 189]
[Pg 189]

At about that time (1833), the Duke of Richmond proved that the fertilizing value of bones resided not in the gelatin, nor in the calcium, but in the phosphoric acid. Thus, he confirmed what Théodore de Saussure had said in 1804, that “we have no reason to believe” that plants can exist without phosphorus. Unknowingly at first, the farmer had supplied this element by means of the organic fertilizers he used: manure, excrements, bones, and horns. Now, with the value of phosphorus known, a search began for mineral phosphates to be applied as fertilizers. Jean Baptiste Boussingault (1802-1887), an agricultural chemist in Lyons, traveled to Peru to see the guano deposits. Garcilaso de la Vega (ca. 1540 to ca. 1616) noted in his history of Peru (1604) that guano was used by the Incas as a fertilizer. Two hundred years later, Alexander von Humboldt revived this knowledge, and Humphry Davy wrote about the benefits of guano to the soil. Yet, the application of this fertilizer developed only slowly, until Justus Liebig sang its praise. Imports into England rose and far exceeded those into France where, between 1857 and 1867, about 50,000 tons were annually received.
The other great advance in the use of phosphatic plant nutrients started with Liebig’s recommendation (1840) to treat bones with sulfuric acid for solubilization. This idea was not entirely new; since 1832, a production of a “superphosphate” from bones and sulfuric acid had been in progress at Prague. At[Pg 186] Rothamsted in 1842, John Bennet Lawes obtained a patent on the manufacture of superphosphate. Other manufactures in England followed and were successful, although James Muspratt (1793-1886) at Newton lost much time and “some thousands of pounds” on Liebig’s idea of a “mineral manure.”

Figure 8.—Florida land-pebble phosphate mining. (From
Carroll D. Wright, The Phosphate Industry of the United States ...,
plate facing page 58.)
In a reciprocal action, more phosphate application to soils stimulated increasing research on the conditions and reactions obtaining in the complex and varying compositions called soil. The findings of bacteriologists made it clear that physics and chemistry had to be amplified by biology for a real understanding of fertilizer effects. After 1900, for example, Julius Stoklasa (1857-1936) pointed out that bacterial action in soil solubilizes water-insoluble phosphates and makes them available to the plants.[26]

Figure 9.—Florida river-pebble phosphate mining. (From
Carroll D. Wright, The Phosphate Industry of the United States ...,
plate facing page 64.)
From Inorganic to Organic Phosphates
By the middle of the 19th century, the source of phosphorus in natural phosphates and the chemistry of its oxidation products had been established. The main difficulty that had to be overcome was that these oxidation products existed in so many forms, not only several stages of oxidation, but, in addition, aggregations and condensations of the phosphoric acids. Once the fundamental chemistry of these acids was elucidated, the attention of chemists and physiologists turned to the task of finding the actual state in which phosphorus compounds were present in the organisms. It had been a great advance when it had been shown that plants need phosphates in their soil. This led to the next question concerning the materials in the body of the plant for which phosphates were being used and into which they were incorporated. Similarly, the knowledge that animals attain their phosphates from the digested plant food called, in the next step of scientific inquiry, for information on the nature of phosphates produced from this source.The method used in this inquiry was to subject anatomically separated parts of the organisms to chemical separations. The means for such separations had to be more gentle than the strong heat and destructive chemicals that had been considered adequate up to then. The interpretation of the new results naturally relied on the general advance of chemistry, the development of new methods for isolating substances of little stability, of new concepts concerning the arrangements of atoms in the molecules, and of new apparatus to measure their rates of change.[Pg 188]

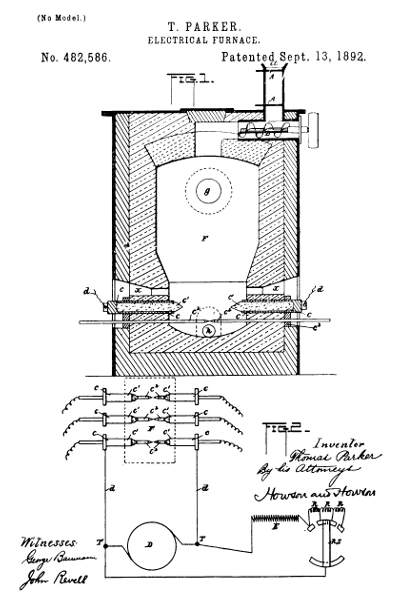
Figure 10.—Electric furnace for producing elemental
phosphorus, invented by Thomas Parker of Newbridge, England, and
assigned to The Electric Construction Corporation of the same place. The
drawing is part of United States patent 482,586 (September 13, 1892).
The furnace was patented in England on October 29, 1889 (no. 17,060); in
France on June 23, 1890 (no. 206,566); in Germany on June 17, 1890 (no.
55,700); and in Italy on October 23, 1890 (no. 431). The following
explanation is cited from the U.S. patent:
Figure 1 [shown here] is a vertical section of the furnace, and Fig. 2 is a diagram to illustrate the means for regulating the electro-motive force or quantity of current across the furnace.
F is the furnace containing the charge to be treated. It has an inlet-hopper at a, with slides AA, by which the charge can be admitted without opening communication between the interior of the furnace and the outer air.
B is a screw conveyer by which the charge is pushed forward into the furnace.
c´c´ are the electrodes, consisting of blocks or cylinders or the like of carbon fixed in metal socket-pieces c c, to which the electric-circuit wires d from the dynamo D are affixed. The current, as aforesaid, may be either continuous or alternating. c2c2 are rods of metal or carbon, which are used to establish the electric circuit through the furnace, the said rods being inserted into holes in conductors c3 (in contact with the socket-pieces c) and in the furnace, as shown.
g is the outlet for the gas or vapor, h the slag-tap hole, and x the opening for manipulating the charge, the said openings being closed by clay or otherwise when the furnace is at work.
I use coke or other form of carbon in the charge between the electrodes c´, the said coke being in contact with the said electrodes, so that complete incandescence is insured.
A means for varying the electro-motive force or quantity of current across the furnace with the varying resistance of the charge is illustrated by the diagram, Fig. 2. c´ c2 indicate the electrodes in the furnace, as in Fig. 1, and D is the dynamo and T its terminals. E represents the exciting-circuit. R R are resistances, and R S is the resistance-switch, which is operated to put in more or less resistance at R as the resistance of the charge in the furnace lessens or increases. This switch may be automatically operated, and a suitable arrangement for the purpose is a current-regulator such as is described in the specification of English Letters Patent No. 14,504, of September 14, 1889, granted to William Henry Douglas and Thomas Hugh Parker.

Figure 11.—Dipping of matchsticks in France, about 1870.
The frame which holds the matches so that one end protrudes at the
bottom, is lowered over a pan containing molten sulfur. The
sulfur-covered matches are then dropped into a phosphorous paste. See
figure 12. (From Figuier, Merveilles de l’industrie, volume 3, 1874,
page 575.)

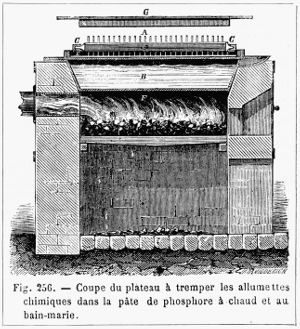
Figure 12.—Pan for dipping matchsticks into phosphorus
paste, about 1870. The letters on the picture are: A, matches; B, water
bath; C, frame; D, plate; E, phosphorus paste; F, oven. The phosphorus
paste of Böttger, 1842, contained 10 phosphorus, 25 antimony sulfide,
12.5 manganese dioxide, 15 gelatin. According to Figuier (page 579), R.
Wagner substituted lead dioxide for the manganese dioxide. (From
Figuier, volume 3, 1874, page 576.)
In the system of chemistry, as it developed in the first half of the
19th century, the new development can be characterized as the turn from
inorganic to organic phosphates, from the substance of minerals and
strong chemical interactions to the components in which phosphate groups
remained combined with carbon-containing substances.