The important phosphorus compounds in organisms are much more complex
than the simple salts, to which Nietzsche attributed such influence on
man’s character. Long before he wrote, it was known that phosphoric acid
combines not only with inorganic bases to form salts, but with alcohols
to form esters. In the middle of the 19th century, Théophile Juste
Pelouze (1807-1867) extended this knowledge to an ester of glycerol.
This proved to be significant in several respects. Glycerol had been
shown by Michel Chevreul (1786-1889) as the substance in fats that is
released in the process of soap boiling, when the fatty acids are
converted into their salts. That it has the nature of an alcohol had
been demonstrated by Marcellin Berthelot. Instead of one “alcoholic”
hydroxyl group, OH, like ethanol (the alcohol of fermentation), or two
hydroxyl groups (like ethylene glycol), glycerol contains three such
groups. It was the only “natural” alcohol known at that time. That this
alcohol would combine with phosphoric acid could be predicted, but that
the ester, as obtained by Pelouze, still contained free acidic functions
and formed a water-soluble barium salt was a new experience.
[Pg 190]
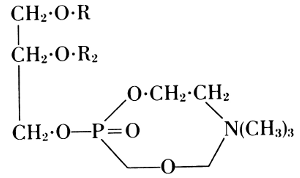
This formula was not quite correct. Richard Willstätter showed that an
internal neutralization takes place between the amino group and the free
acidic residue. This is expressed in his lecithin formula of 1918.
 When the aim was to distill elementary phosphorus out of an organic
material, it did not matter whether this was fresh or putrified. For
obtaining lecithin out of egg yolk and similar materials, it was
essential to use it in fresh condition. Otherwise, enzymes would have
decomposed it. Through more recent work, four enzymes have been
separated, which act specifically in decomposing lecithin. Enzyme A
removes one fatty acid and leaves a complex residue, called
lysolecithin, intact. Enzyme B attacks this residue and splits off the
remaining fatty acid group from it, enzyme C liberates only the choline
from lecithin, and enzyme D opens lecithin at the ester bond between
glycerol and phosphoric acid. This is shown in the following diagram.
When the aim was to distill elementary phosphorus out of an organic
material, it did not matter whether this was fresh or putrified. For
obtaining lecithin out of egg yolk and similar materials, it was
essential to use it in fresh condition. Otherwise, enzymes would have
decomposed it. Through more recent work, four enzymes have been
separated, which act specifically in decomposing lecithin. Enzyme A
removes one fatty acid and leaves a complex residue, called
lysolecithin, intact. Enzyme B attacks this residue and splits off the
remaining fatty acid group from it, enzyme C liberates only the choline
from lecithin, and enzyme D opens lecithin at the ester bond between
glycerol and phosphoric acid. This is shown in the following diagram.
Several fatty acids can be present in lecithin from various sources:
palmitic and oleic acid, besides the stearic acid which at first had
been thought the only one involved. In another group of extracts from
brain or nerve tissue, amino-ethanol H2NCH2CH2OH is found
instead of the choline of lecithin. The variations include the alcohol,
to which the fatty acids and choline phosphate are attached, for
example, glycerol can be replaced by the so-called meat-sugar, inositol,
which has six hydroxyl groups in its hexagon-shaped molecule
[Pg 192]C6H6(OH)6.
 The generally similar behavior of these phosphate-and fat-containing
substances was emphasized by Ludwig Thudichum (1829-1901). He coined the
name phosphatides for this group of substances from seeds and
nerves.[29] His work on the phosphates in brain substance aroused
particular interest. When William Crookes drew his highly imaginative
picture of an “evolution” of the chemical elements, he put into it
“phosphorus for the brain, salt for the sea, clay for the solid
earth....”[30] But
phosphatides occur in many places of organisms, in
bacteria, in leaves and roots of plants, in fat and tissues of animals.
And where phosphatides are found, there are also enzymes that
specifically act on them. They are called phosphatases to imply that
they split the phosphatides. In addition, enzymes are present, which
transfer phosphate groups from one compound to another. They are more
abundant in seeds of high fat content than in the more starch-containing
seeds, but even potatoes and orange juice have phosphatases.[31]
The generally similar behavior of these phosphate-and fat-containing
substances was emphasized by Ludwig Thudichum (1829-1901). He coined the
name phosphatides for this group of substances from seeds and
nerves.[29] His work on the phosphates in brain substance aroused
particular interest. When William Crookes drew his highly imaginative
picture of an “evolution” of the chemical elements, he put into it
“phosphorus for the brain, salt for the sea, clay for the solid
earth....”[30] But
phosphatides occur in many places of organisms, in
bacteria, in leaves and roots of plants, in fat and tissues of animals.
And where phosphatides are found, there are also enzymes that
specifically act on them. They are called phosphatases to imply that
they split the phosphatides. In addition, enzymes are present, which
transfer phosphate groups from one compound to another. They are more
abundant in seeds of high fat content than in the more starch-containing
seeds, but even potatoes and orange juice have phosphatases.[31]
Thus, from phosphatides, phosphoric acid is generated, and they could also be called phosphagens. Since 1926, however, the name phosphagens has been reserved for a group of organic substances that release their phosphoric acid very readily. The link between phosphorus and carbon is provided by oxygen in the phosphatides, by nitrogen in the phosphagens. In vertebrates, the basis for the phosphoric acid is creatine, whereas invertebrates have arginine instead.
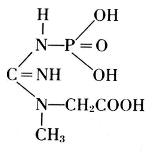 Creatine Phosphate
Creatine Phosphate
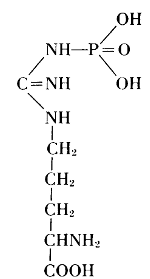 Arginine phosphate
Arginine phosphate
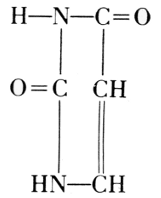
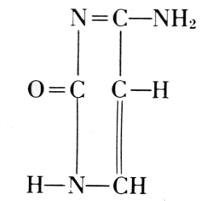
This was also true for the methods of chemical degradation, carried out in order to find the components of nucleins in their highest state of natural complexity. It was learned for example, that the special kind of carbohydrate present in nucleins was very susceptible to change under the conditions of hydrolysis by acids. Phoebus Aaron Theodor Levine (1869-1940), therefore, used the digestion by a living organism. With E. S. London, he introduced a solution of nucleic acid into, e.g., the gastrointestinal segment of a dog through a gastric fistula and withdrew the product of digestion through an intestinal fistula. Fortunately, the products obtained in such degradations were not new in themselves. The carbohydrate in this nucleic acid proved to be identical with D-ribose, which Emil Fischer had artificially made from arabinose and named ribose to indicate this relationship (1891). The nitrogenous products of the degradation were identical with substances previously prepared in the long study of uric acid. In the course of this study, Emil Fischer established uric acid and a number of its derivatives as having the elementary skeleton of what he called “pure uric acid,” abbreviated to purine. Out of Adolf Baeyer’s work on barbituric acid came the knowledge of pyrimidine and its derivatives.
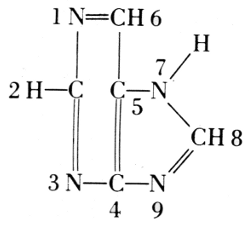
 From these findings, together with what Oswald Schmiedeberg (1838-1921)
had established concerning the presence of four phosphate groups in the
molecule (1899), Robert Feulgen (1884-1955) constructed the following
scheme of a nucleic acid. Feulgen’s formula of 1918 is:
From these findings, together with what Oswald Schmiedeberg (1838-1921)
had established concerning the presence of four phosphate groups in the
molecule (1899), Robert Feulgen (1884-1955) constructed the following
scheme of a nucleic acid. Feulgen’s formula of 1918 is:
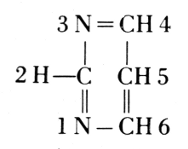
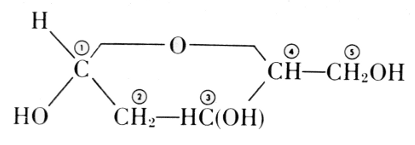 The exact position of phosphoric acid was established after long work
and verified by synthesis.[33]
The exact position of phosphoric acid was established after long work
and verified by synthesis.[33]
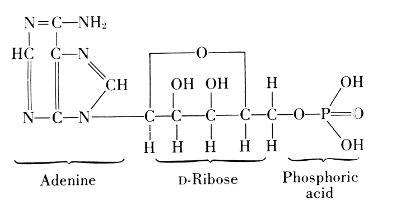
A compound of adenine, ribose, and phosphoric acid was found in yeast, blood, and in skeletal muscle of mammals. From 100 grams of such muscle, 0.35-0.40 grams of this compound were isolated. If the muscle is at rest, the compound contains three molecules of phosphoric acid, linked through oxygen atoms. It was named adenosine triphosphate or adenyltriphosphoric acid,[34] usually abbreviated by the symbol ATP. It releases one phosphoric acid group very easily and goes over in the diphosphate, ADP, but it can also lose 2 P-groups as pyrophosphoric acid and leave the monophosphate, AMP.
 This change of ATP was considered to be the main source of energy in
muscle contraction by Otto Meyerhof.[35] The corresponding derivatives
of guanine, cytosine, and uracil were also found, and they are active in
the temporary transfer of phosphoric acid groups in biological
processes.
This change of ATP was considered to be the main source of energy in
muscle contraction by Otto Meyerhof.[35] The corresponding derivatives
of guanine, cytosine, and uracil were also found, and they are active in
the temporary transfer of phosphoric acid groups in biological
processes.
Thus, the study of organic phosphates progressed from the comparatively simple esters connected with fatty substances of organisms to the proteins and the nuclear substances of the cell. The proportional amount of phosphorus in the former was larger than in the latter; the actual importance and function in the life of organisms, however, is not measured by the quantity but determined by the special nature of the compounds.[Pg 195]


 [Pg 196]
[Pg 196]


 [Pg 197]
The study of this function is the newest phase in the history of
phosphorus and represents the culmination of the previous efforts. This
newest phase developed out of an accidental discovery concerning one of
the oldest organic-chemical industries, the production of alcohol by the
fermentative action of yeast on sugar. A transition of carbohydrates
through phosphate compounds to the end products of the fermentation
process was found, and it gradually proved to be a kind of model for a
host of biological processes.
[Pg 197]
The study of this function is the newest phase in the history of
phosphorus and represents the culmination of the previous efforts. This
newest phase developed out of an accidental discovery concerning one of
the oldest organic-chemical industries, the production of alcohol by the
fermentative action of yeast on sugar. A transition of carbohydrates
through phosphate compounds to the end products of the fermentation
process was found, and it gradually proved to be a kind of model for a
host of biological processes.
Specific phosphates were thus found to be indispensable for life. In reverse, the wrong kind of phosphates can destroy life. As a result, an important part of the new phase in phosphorus history consisted in the study—and use—of antibiotic phosphorus compounds.
[Pg 190]

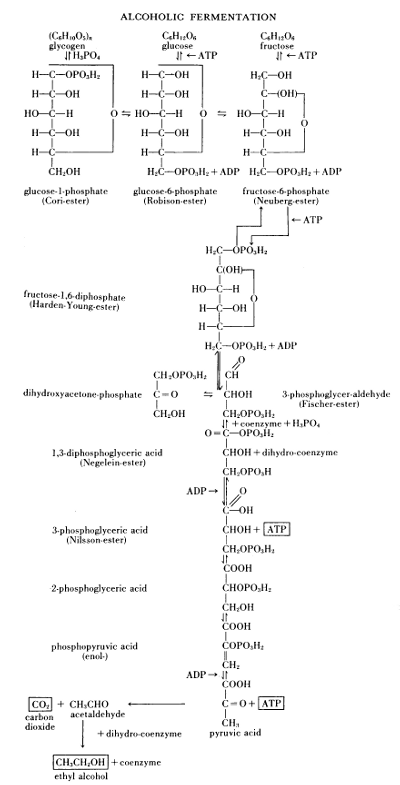
Figure 13.—Survey of alcoholic fermentation, 1951. The
“well-known scheme of alcoholic fermentation” according to Albert Jan
Kluyver (1888-1956), presented before the Society of Chemical Industry
in the Royal Institution, March 7, 1951. In Chemistry & Industry,
1952, page 136 ff., Kluyver restates that “... the fermentation of one
molecule of glucose is indissolubly connected with the formation of two
molecules of adenosine triphosphate (ATP) out of two molecules of
adenosine diphosphate (ADP).”
[Pg 191]
Shortly after this experience had been gained, it became valuable for
understanding the chemical nature of a new substance extracted from a
natural organ. This substance was named lecithin by its discoverer,
Nicolas Théodore Gobley[27] (1811-1876), because he obtained it from egg
yolk (in Greek, lékidos). He used ether and alcohol for this
extraction. Had he used water and mineral acid instead, he would not
have found lecithin, but only its components. As Gobley and, slightly
later, Oscar Liebreich (1839-1908), subjected lecithin to treatment with
boiling water and acid, they separated it into three parts. One of them
was the glycerophosphoric acid of Pelouze, the second was the well-known
stearic acid of Chevreul, but the third was somewhat mysterious. This
third substance was the same as one previously noticed when nerves had
been subjected to an extraction by boiling water and acid and,
therefore, called nerve-substance or neurine. Adolf Friedrich Strecker
(1822-1871) established the identity of this neurine with a product he
had extracted from bile and which went under the name of choline.
Adolphe Wurtz (1817-1884) succeeded in synthesizing this substance from
ethylene oxide, CH2.O.CH2 and trimethylamine N(CH3)3.[28] Thus, all
three parts were identified, and Strecker put them together to construct
a chemical formula for lecithin, glycerophosphoric acid combined with a
fatty acid and with choline (a hydrate of neurine).
| { | OH | } | ||
| N | (CH3)3 | Choline | ||
| C2H4O | ||||
| C18H33O2 | } | HO | } | ||
| PO | |||||
| C16H31O2 | C3H5O | ||||
| Fatty Acids | Glycerophosphate | ||||
| '—————v————' | |||||
| Lecithin | |||||
| according to Strecker | |||||

Lecithin (1918)
| Enzymatic Splitting of Lecithins | ||
|---|---|---|
| Enzyme | Substrate | Products |
| A | Lecithin | Lysolecithin and fatty acids. |
| B | Lysolecithin | Glycero-phospho-choline and fatty acids. |
| C | Lecithin | Phosphatidic acid and choline. |
| D | Lecithin | Phosphoryl choline and diglyceride. |

Figure 14.—Eduard Buchner (1860-1917) received the Nobel
Prize in Chemistry for his discovery of cell-free fermentation, the
first step in finding the role of phosphate in fermentations (1907).
Thus, from phosphatides, phosphoric acid is generated, and they could also be called phosphagens. Since 1926, however, the name phosphagens has been reserved for a group of organic substances that release their phosphoric acid very readily. The link between phosphorus and carbon is provided by oxygen in the phosphatides, by nitrogen in the phosphagens. In vertebrates, the basis for the phosphoric acid is creatine, whereas invertebrates have arginine instead.
 Creatine Phosphate
Creatine Phosphate
 Arginine phosphate
Arginine phosphate
Nuclein and Nucleic Acids
All parts of an organism are essential for life. Only with this in mind does it make sense to say that the most important part of the cell is its nucleus. From the nuclei of cells in pus and in salmon sperm, Johann Friedrich Miescher (1811-1887) obtained a peculiar kind of substance, which he named nuclein (1868). Its phosphate content was easily discovered, but to find the exact proportions and the nature of the other components required special methods of separation from phosphatides and other proteins. It was difficult to develop such methods at a time when little was known about the properties, and particularly[Pg 193] the stability, of a nuclein. For preparing nuclein from yeast cells, Felix Hoppe-Seyler (1825-1895) described the following details: Yeast is dispersed in water to extract soluble materials, like salts or sugars. After a few hours, the insoluble material is separated, washed once more with water, and then extracted with a very dilute solution of sodium hydroxide. The slightly alkaline solution, freed from insoluble residues, is slowly added to a weak hydrochloric acid. A precipitate forms which is separated by filtration, washed with dilute acid, then with cold alcohol, and finally extracted by boiling alcohol. The dried residue is the nuclein.[32] It contains six percent phosphorus. A little more washing with water, a slightly longer treatment with acid or alcohol gives products of lower phosphorus content. Many experimental variations were necessary to establish the procedure that leads to purification without alteration of the natural substance.This was also true for the methods of chemical degradation, carried out in order to find the components of nucleins in their highest state of natural complexity. It was learned for example, that the special kind of carbohydrate present in nucleins was very susceptible to change under the conditions of hydrolysis by acids. Phoebus Aaron Theodor Levine (1869-1940), therefore, used the digestion by a living organism. With E. S. London, he introduced a solution of nucleic acid into, e.g., the gastrointestinal segment of a dog through a gastric fistula and withdrew the product of digestion through an intestinal fistula. Fortunately, the products obtained in such degradations were not new in themselves. The carbohydrate in this nucleic acid proved to be identical with D-ribose, which Emil Fischer had artificially made from arabinose and named ribose to indicate this relationship (1891). The nitrogenous products of the degradation were identical with substances previously prepared in the long study of uric acid. In the course of this study, Emil Fischer established uric acid and a number of its derivatives as having the elementary skeleton of what he called “pure uric acid,” abbreviated to purine. Out of Adolf Baeyer’s work on barbituric acid came the knowledge of pyrimidine and its derivatives.

Figure 15.—Albrecht Kossel (1853-1927) received the
Nobel Prize in Medicine and Physiology in 1910 for his work on nucleic
substances, which contain a high proportion of phosphorus. The chemical
bonds of this phosphorus in the molecules of nucleic substances were
determined in later work. (Photo courtesy National Library of Medicine,
Washington, D.C.)
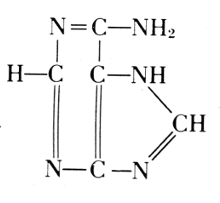
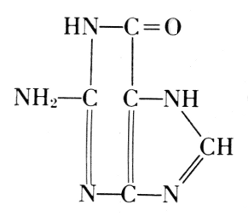
Phosphoric acid—Carbohydrate—Guanine
Phosphoric acid—Carbohydrate—Cytosine
Phosphoric acid—Carbohydrate—Thymine
Phosphoric acid—Carbohydrate—Adenine
Of the four basic components on the right, thymine occurs in the
nucleic
acid from the thymus gland. Yeast contains uracil instead. The
difference between these two bases is one methyl group: thymine is a
5-methyluracil. In all of these basic substances, the structure of ureaPhosphoric acid—Carbohydrate—Cytosine
Phosphoric acid—Carbohydrate—Thymine
Phosphoric acid—Carbohydrate—Adenine
NH2
/
C=O
\
NH2
is involved, and they form pairs of oxidized and reduced states:
[Pg 194]| Purine | Pyrimidine | |
| (reduced) Adenine | + | (oxidized) Thymine |
| (oxidized) Guanine | + | (reduced) Cytosine |
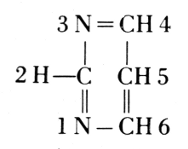
Pyrimidine

Purine

Adenine

Guanine

Uracil

Cytosine
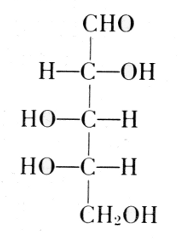
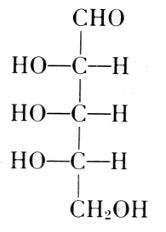
The carbohydrate is ribose or deoxyribose.

Arabinose

l-Ribose
Fischer and Piloty, 1891

Deoxyribose
A compound of adenine, ribose, and phosphoric acid was found in yeast, blood, and in skeletal muscle of mammals. From 100 grams of such muscle, 0.35-0.40 grams of this compound were isolated. If the muscle is at rest, the compound contains three molecules of phosphoric acid, linked through oxygen atoms. It was named adenosine triphosphate or adenyltriphosphoric acid,[34] usually abbreviated by the symbol ATP. It releases one phosphoric acid group very easily and goes over in the diphosphate, ADP, but it can also lose 2 P-groups as pyrophosphoric acid and leave the monophosphate, AMP.

Thus, the study of organic phosphates progressed from the comparatively simple esters connected with fatty substances of organisms to the proteins and the nuclear substances of the cell. The proportional amount of phosphorus in the former was larger than in the latter; the actual importance and function in the life of organisms, however, is not measured by the quantity but determined by the special nature of the compounds.[Pg 195]

Figure 16.—Otto Meyerhof (1884-1951) received one-half
of the Nobel Prize in Medicine and Physiology in 1922 for his discovery
of the metabolism of lactic acid in muscle, which involves the action of
phosphates, especially adenosine duophosphates. (Photo courtesy
National Library of Medicine, Washington, D.C.)


Figure 17.—Arthur Harden (1865-1940), left, and Hans A.
S. von Euler-Chelpin (b. 1875), right, shared the Nobel Prize in
Chemistry in 1929. Harden received it for his research in fermentation,
which showed the influence of phosphate, particularly the formation of a
hexose diphosphate. Euler-Chelpin received his award for his research in
fermentation. He found coenzyme A which is a nucleotide containing
phosphoric acid.

Figure 18.—George de Hevesy (b. 1885) received the Nobel
Prize in Chemistry in 1943 for his research with isotopic tracer
elements, particularly radiophosphorus of weight 32 (ordinary phosphorus
is 31).


Figure 19.—Carl F. Cori (b. 1896) and his wife, Gerty T.
Cori (1896-1957) received part of the Nobel Prize in Medicine and
Physiology in 1947 for their study on glycogen conversion. In the course
of this study, they identified glucose 1-phosphate, now usually referred
to as “Cori ester,” and its function in the glycogen cycle. (Photo
courtesy National Library of Medicine, Washington, D.C.)
Specific phosphates were thus found to be indispensable for life. In reverse, the wrong kind of phosphates can destroy life. As a result, an important part of the new phase in phosphorus history consisted in the study—and use—of antibiotic phosphorus compounds.